A quarter century ago when I was in college, it was the fashion of the day to put posters on your dorm room wall. For a lot of the straight guys, that meant posters of improbably hot babes… cheerleaders and models and such. Other guys put up posters of football and basketball players; by process of elimination, these would not seem to have been other straight guys.
This is one of the posters I put up:
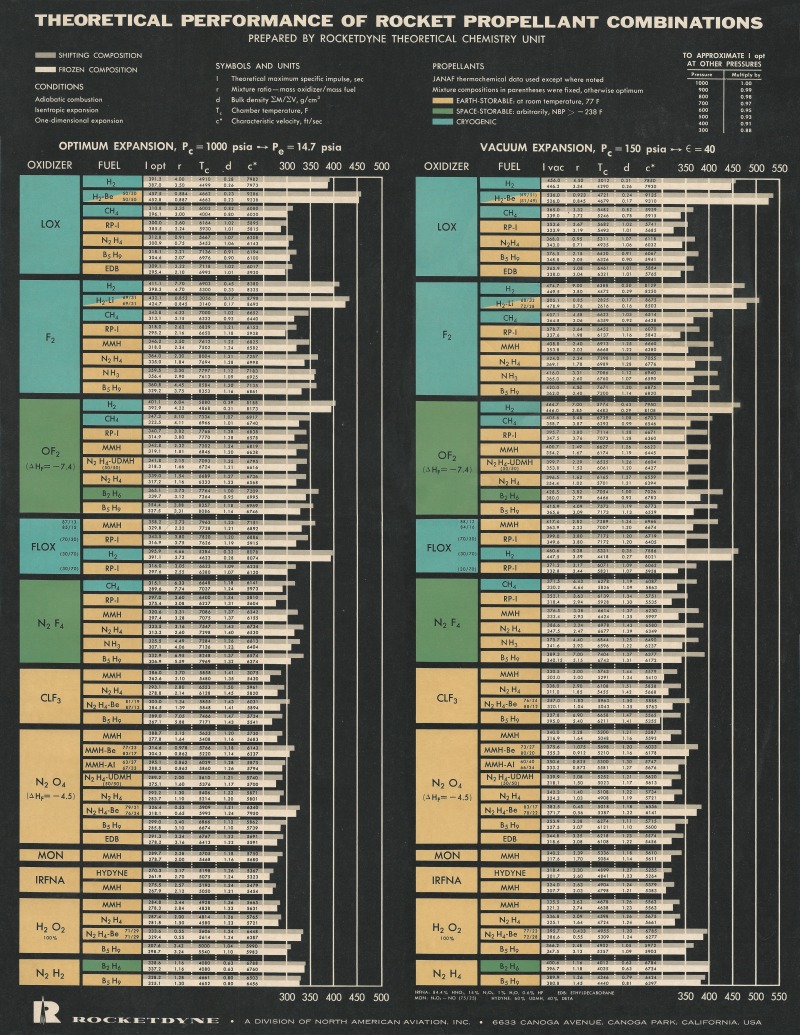
I picked up this “Theoretical Performance of Rocket Propellant Combinations” poster by Rocketdyne at the Dayton Air Show sometime around 1989, and now it’s in pretty rough shape. It was stuck to a dorm wall with blu-tack and tape, and torn and taped back up; it survived college to wind up stuck to the walls at various places of aerospace employment and followed me all over the continent. I’ve finally gotten around to scanning the thing, and will expend some effort in fixing it up.
Never mind semi-naked hotties, *this* was the thing to have on your wall. This and the cutaway diagram of the starship Enterprise and the posters of nuclear mushroom clouds were *way* better. Yes, I was the coolest.
Some time ago I scored two other variants of the Propellant Performance poster off of ebay. The one below dates from 1964 and is printed on what has got to be the worst paper ever created… no flexibility whatsoever. I have books centuries old that have paper far less brittle that this thing. Fortunately I got it scanned and am well on the way to restoring the image.
And the third one? Frakked if I know where it is. I assume one of my cats has hidden it for some nefarious purpose.